GEOB 407 Project
April 17, 2020
Abstract
Alliaria petiolata, known as garlic mustard, has colonized the North American East Coast and Midwestern regions at relatively high concentrations, and poses dangers to forest biodiversity and productivity. In this study, we conduct a meta-analysis on a small set of available experimental literature on Alliaria petiolata to try understand the currently stunted invasion into lower British Columbia. Using 24 matrices based on garlic mustard populations found in the North American Midwestern region, we simulated the theoretical population growth rates in lower British Columbia based on changes in temperature, precipitation, sunlight, and powdery mildew infection. Based on our results, we found that the population growth rates of garlic mustard in lower B.C. are slightly lower than those in the Midwest, with temperature being the primary driver of the limited distribution. Although climate change is decreasing the number of cold days needed to break seed dormancy, local adaptations may allow populations to continue to survive in lower B.C.. Where precipitation and sunlight made minimal impacts, the absence of powdery mildew fungal infection was found to substantially increase growth rates in lower B.C.. Due to the strong impacts powdery mildew on garlic mustard fitness, it should be further researched as a possible biocontrol agent, especially before garlic mustard inevitably becomes invasive in the lower B.C. region.
Introduction
It has been found that species around the world are shifting ranges due to anthropogenic climate change, and this is true for invasive species as well (Parmesan & Yohe 2003). Understanding population dynamics of invasives can help find contributing reasons for exponential population growth and help predict the mortality needed to induce a slowdown (Pardini et al. 2009). Using those variables, we can model population dynamics of species in future habitats to learn the degree of potential invasion. It is important to understand the potential impacts of these invasive species before they arrive so that resource managers can take appropriate mitigative action, especially if establishment is inevitable due to climatic factors.
Alliaria petiolata, commonly known as the garlic mustard, is an herbaceous biennial plant that dominates the forest understory and out-competes native vegetations (Vaughn & Berhow 1999). Originally from western Eurasia, it was introduced in the mid-19th century by colonists for medicinal and culinary purposes (Rodgers et al. 2008). By 1990, it had established in three Canadian provinces, as well as 27 midwestern and northeastern states in the United States (Nuzzo 1991). This fast-spreading colonization is supported by its high energy investment in reproduction, it develops an autogamous breeding system and is capable of producing an annual seed rain of 15,000 seeds per m2 (Anderson et al. 1996).
Researchers are most worried over the species’ ability to exclude native plants through allelopathic inhibition. Garlic mustard tissue has been found to contain levels of cyanide that decreases native plant growth and changes soil nutrient cycling, resulting in lower biodiversity and productivity (Rodgers et al. 2008, Cipollini & Gruner 2006, Prati & Bossdorf 2004, Stinson et al. 2007). The decline in biodiversity indices is concerning; as forest understories become dominated by garlic mustard, forest ecosystems will become less resilient to environmental change and disturbance. The plant’s allelopathy enables it to reduce resource competition in the area it establishes in, which is likely a major contributing factor in its geographically widespread invasion.
Alliaria petiolata has colonized the North American East Coast and Midwestern regions at relatively high concentrations, and is present in the Pacific Northwest at lower concentrations (Welk et al. 2002). In British Columbia, small infestations of the plant have been found in Victoria, Saanich, and in Metro Vancouver (City of Victoria 2010, City of Burnaby n.d.). Based on a temperature and precipitation climate model, lower British Columbia is predicted to become a hotspot for garlic mustard in the future (Welk et al. 2002). While it is tolerant of many habitat conditions, it is often found in damp shaded soils in disturbed deciduous woodlands, particularly near river-associated habitats (Nuzzo 1991, Cavers et al. 1978). These environmental conditions are abundant in lower British Columbia, and yet the infestation has not reached levels seen in the Midwest.
Other factors that seemed to have an influence on the fitness of garlic mustard include soil acidity and elevation. Soil acidity has been found to have a very strong negative relationship with dry biomass, although this will not affect reproduction because garlic mustard is an “obligate biennial” that will produce seeds after winter regardless of its size (Anderson & Kelly 1995, Nuzzo 1993a). Plant occurrence probability is strongly affected by increasing elevation, decreasing by 50% between 0 and 300m (Urbanowicz et al. 2019). However, we could not use this relationship because the mean elevations of lower British Columbia and the Midwest are relatively similar.
In this study, we will conduct a meta-analysis on a small set of available experimental literature on Alliaria petiolata to try understand the currently stunted invasion into lower British Columbia. We looked for experiments testing variables that were noticeably different between lower British Columbia and the Midwest. By applying the vital rate impact of those variables to Midwestern plant population matrices, we hoped to find a variable that would reduce population growth rates enough to explain why garlic mustard populations have not invaded lower British Columbia as aggressively. We also ran an elasticity analysis to find which stage transition contributed the most to changes in population growth rates.
We selected four studies that experimented on variables with obvious differences between lower British Columbia and the Midwest: temperature, powdery mildew infection, precipitation, and light availability. We expected that based on these variables, we would see smaller population growth rates in lower B.C. because the current invasion of garlic mustard has not reached levels seen in the Midwest. Of the experimental variables we used, we believed temperature would be the greatest obstacle to the invasive establishment of garlic mustard because of the drastic winter and spring temperature differences between Southern B.C. and North American Midwestern winters (Kottek et al. 2006). The differences in late winter and early spring temperatures are drastic between the two geographic areas, and was likely the largest contributing factor in the distribution difference among the three we selected.
Powdery mildew infection is present in British Columbia, but we only found papers that indicate B.C. wasabi plants have been infected, rather than garlic mustard (Betz & Punja, 2018). Instead, it has infected many plant populations in the Midwest, particularly in southwestern Ohio (Ciola & Cipollini 2011). If anything, the smaller presence of the fungal infection in British Columbia would increase its likelihood of invasion. We also selected light availability due to the approximately 10° difference in latitude between the two regions, which would result in differing daylight hours depending on the season. While there is a difference, we did not think that it would be significant enough to have a bigger impact than temperature. Vancouver is known to be quite wet in winter and spring months, but it has been found that increasing precipitation is correlated with increasing population growth (Merow et al. 2017). This means the precipitation variable would result in higher population growth rates in lower B.C. than the midwest.
Methods
We collected population matrix data of Alliaria petiolata from the Compadre database, where we found 50 different matrices from 10 locations across the Midwest and sampled from different years (2005 to 2008). We imported all of the matrices to R studio and constructed the “seeds, rosettes and flowering plants” 3-life-stages matrix (Evans et al. 2012). Using only growing populations, we coded loops to change the vital rates accordingly and calculate the new population growth rate after changes. Growth rates had to be greater than 1, since populations with shrinking populations are unlikely to colonize new areas and are not of critical importance. Finally, we used ggplot to present our results. We also compiled the vital rates that had the highest elasticity in each matrix in a bar graph (Figure 3).
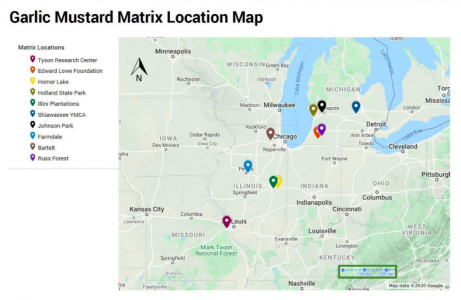
Figure 1. Distribution of Midwestern garlic mustard population matrices downloaded from Compadre Plant Matrix Database. We used Chicago as our reference location for sunlight and precipitation comparisons because it sits at the center of the range of our population samples.
To calculate the necessary changes in plant stage survival, we consulted four articles of experimental research on Alliaria petiolata. Garlic mustard in Ohio was found to produce half as many seeds when intermediately or severely infected by powdery mildew fungal infection (Enright & Cipollini 2007). To reflect this in the matrices, we halved the flowering plant to seed transition vital rate for each matrix.
We compared April daylight hours in Vancouver, B.C. and Chicago, Illinois to calculate the effects of light on seedling survival. Chicago was used because the city is located near the center of the matrix sample distribution in the Midwest. Garlic mustard seedling establishment was recorded in May, so we focused on April light hours. Based on cumulative April 2020 daylength hour data from TimeAndDate.com, we found that Chicago will experience 3.01% less lighthours than Vancouver. According to an experiment on the effects of light on garlic mustard, decreasing light by one standard deviation results in a 0.559 standard deviation decrease in rosette survival (Phillips-Mao et al. 2014). As a result, the rosette survival should be (0.0301 * 0.559 * 0.341) = 0.00574 more in Vancouver. We adjusted each rosette survival rate accordingly.
We compared winter and early spring temperatures of various cities in lower B.C. and the Midwest to estimate the effects of temperature on seed germination. Seeds subjected to more than 90 “cold treatment days” (6°C to -1°C) will statistically have a germination rate greater than zero (Raghu & Post 2008). Using temperature data from the United States National Oceanic and Atmospheric Administration for 21 stations in lower B.C. (20 in B.C. and 1 in Washington) and 19 stations across the Midwest (1 in Illinois, 14 in Michigan, 1 in Ohio, and 3 in Ontario) between September 22, 2018 to March 31, 2019 (to generally align with winter), we calculated the number of days each station experienced temperatures below 6°C (meaning they have received or exceeded the cold treatment). Zero of the B.C. stations had enough cold days to allow for a significant portion of seed germination. Meanwhile, 89.4% of the Midwestern stations had enough cold days for a significant portion of seed germination. As a result, we reduced the seed to rosette vitality rate to 0 for plants in the Vancouver area.
We compared May precipitation of various cities in lower B.C. and the Midwest to estimate the effects of precipitation on seed reproduction. Decreasing average precipitation in May by 4.5 cm will cause a decrease in seed production by 10% (Cruden, McClain, and Shrivastava 1996). We used data from the same climate stations as our geographical reference to do the May precipitation comparison (NOAA), and we found that B.C. has 1.8 cm less precipitation than the Midwest in May. This will lower the seed reproduction rate of Alliaria petiolata by 4% (1.8cm / 4.5cm * 10% = 4%).
| Variable | Literature | Adjusted Vital Rate | Rationale | Parameter |
| Temperature | Raghu & Post 2008 | Dormant seeds→ Rosettes | Seeds that have experienced more than 90 days of cold environment (6°C to -1°C) will statistically have a germination rate greater than zero. Temperature data from B.C. stations suggests that B.C. does not have enough cold days to allow for seed germination. | vital rate*0 |
| Mildew Infection | Enright & Cipollini 2007 | Flowering plants→ Dormant seeds | Garlic mustard was found to produce half as many seeds when intermediately or severely infected by powdery mildew fungal infection. No study suggests B.C. plants have been affected by powdery mildew fungus. | vital rate*2 |
| Sunlight | Phillips-
Mao et al. 2014 |
Rosettes→ Flowering plants | Decreasing light by one standard deviation results in a 0.559 standard deviation decrease in rosette survival. Garlic mustard seedling establishment was recorded in May, thus April light hours will make significant change to its growth. Based on day length data from Chicago, the Midwest area was found to experience 3.01% shorter daytime than B.C. | vital rate* 1.00574 |
| Precipitation | Cruden, McClain & Shrivastava 1996 | Flowering plants→ Dormant seeds | Decreasing in average precipitation in May by 4.5 cm will cause decrease in seed production by 10%. B.C has less precipitation (7.5 cm) than Chicago (9.3cm), which leads to 4% reduction in seed production. | vital rate*0.96 |
Table 1. Summary of parameters used to adjust the vital rates.
Results
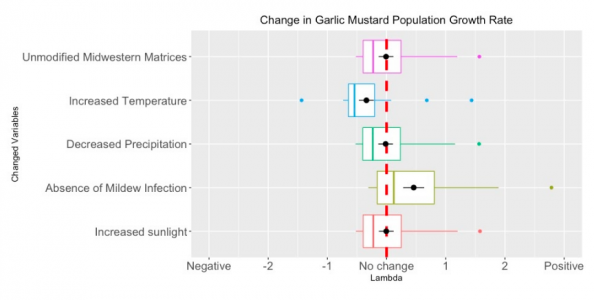
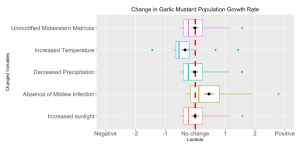
Figure 2. The comparison between changes in population growth rate (lambda) after adjusting the corresponded vital rates. Appropriate Alliaria petiolata matrix (n=24, only growing populations where ƛ>1 were included) vital rates were changed according to environmental condition differences between B.C. province and midwestern North America. The scale of relative changes are indicated by the x-axis, negative values suggest the population growth rate decreased, whereas positive values suggest the population growth rate increased.
| Modification | Mean Population Growth Rate | Change in Mean Population Growth Rate |
| Unmodified Midwestern Matrices | 2.019 | 0 |
| Increased Temperature | 1.722 | -0.296 |
| Decreased Precipitation | 2.012 | -0.00635 |
| Absence of Mildew Infection | 2.709 | +0.691 |
| Increased Sunlight | 2.023 | +0.00469 |
Table 2. Mean population growth rates of growing (ƛ>1) unmodified matrices and matrices that simulate lower British Columbia environmental conditions (n=24).
The decrease in Alliaria petiolata seed germination rate caused by increasing temperature in growth season led to a substantial decrease in population growth rate by 0.296 as shown in Table 2. Figure 2 indicates that the population growth rate rate was not substantially influenced by the precipitation decreases during the reproduction seasons, causing no obvious changes in population growth rate (-0.00635). The increased reproduction of seeds due to lack of mildew infection considerably increased population growth rate by 0.691 (Table 2). On the contrary, increased sunlight did not significantly affect the growth from rosettes to flowering plants, thus we did not observe any notable changes (+0.00469) in the stimulated population growth rate (Figure 2). Between these two variables, the powdery mildew has the potential to increase lambda almost 150 times more than sunlight can. We also calculated the change in population growth rates when all variables were applied at once. To simplify the code, we applied the changes to the mean matrix. The population growth rate of this combined matrix changed from 2.28 to 2.09.
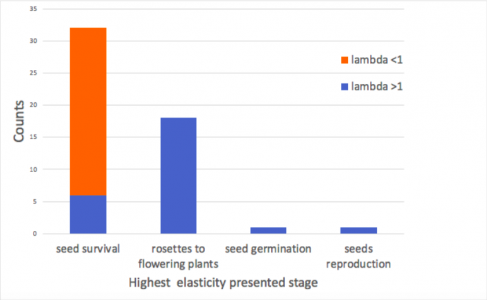
Figure 3. The vital rate distribution of highest elasticity in each matrix (n=50, all matrices were included).
The elasticity analysis result in Figure 3 indicated that in all shrinking Alliaria petiolata populations, seed survival (seeds remain seeds transition) always had the highest elasticity (n=26). However, for the majority (60%) of matrices in growing Alliaria petiolata populations, the rosettes to flowering plant transition had the highest elasticity. The seed germination and seed reproduction transitions were the most elastic rates for one population each (Figure 2). Seed survival was the highest elasticity for 6 growing populations.
Discussion
Our results indicate that increased temperature and decreased precipitation resulted in negative changes in mean population growth (Figure 2). These trends have also been observed in New England populations, where increasing mean temperature in the warmest month corresponded with a decrease in lambda, and decreasing mean May precipitation decreased lambda (Merow et al. 2017). However, Table 2 shows neither variable was substantial enough to swing the growth rates below 1, meaning the B.C. populations will still grow.
When all target vital rates were adjusted at the same time, the growth rates of the mean matrix changed from 2.28 to 2.09. This means that conditions in lower British Columbia are not enough to prevent the establishment of garlic mustard, although the combined effect will slightly decrease the growth rate compared to the Midwestern region. This aligns with our hypothesis, as we predicted the population growth rates would be lower in B.C. because the plant has not colonized the region as aggressively (Welk et al. 2002). As populations are still small, it is possible they are still in the lag phase of exponential growth. It makes sense that the populations are growing, because the plant has established and models have indicated that the plant’s range is shifting northward towards more suitable climatic conditions, like colder temperatures needed for breaking dormancy (Welk et al. 2002, Merow et al. 2017).
While garlic mustard plants require cold wintertime temperatures to germinate, there is a possibility that garlic mustard populations currently present in lower British Columbia have locally adapted to warmer temperatures. Mean minimum temperatures in British Columbia have increased 5.1℃ between 1948 and 2016, and the number of frost days are in decline as well (Vincent et al. 2018). Should this positive trend continue, there will likely be less days with sufficient “cold treatment” to allow for the germination of garlic mustard seeds (Raghu & Post 2008). The “cold treatment” approach suggests that as temperatures continue to rise due to climate change, garlic mustard seeds that disperse into the area may never get the chance to germinate and establish (Stocker et al. 2013). While the Raghu and Post (2008) study suggested that percent germination would statistically be 0% with insufficient cold treatment days, it has been suggested that seedling emergence would continue in low dormancy seeds, which may initially occur in a population at lower rates (Footitt et al. 2018). These low dormancy seeds require less chilling days, and this trait would be selected for as climate change increases global soil temperatures (Footitt et al. 2018). These adaptations may explain the continued presence of garlic mustard in lower B.C. despite higher temperatures. So while our model suggests temperature may be inhibiting a fast-paced invasion of lower B.C., the rate may accelerate in the future as garlic mustard populations adapt to local climatic factors.
Powdery mildew (Erysiphe cruciferarum) has been studied as a possible biocontrol agent; although it does not outright kill the plant, it has the capability of reducing its spread so that native plants may become more competitive (Enright & Cipollini 2007). Powdery mildew has been reported as infecting garlic mustard populations in Indiana and southwest Ohio (Enright & Cipollini 2007). It is highly unlikely that the infection affects all populations in the Midwest, so the increase in growth rate simulated in lower B.C. is likely an overestimation. While there have not been reports of powdery mildew affecting lower B.C. garlic mustard populations, there is a possibility of unknown or future infections because the fungus is known to affect wasabi in the same region (Betz & Punja 2019). As garlic mustard lacks other consistently effective controls, such as herbivory, manual removal, herbicide application, and prescribed fire, powdery mildew could counter the population growth rate effects from other variables, such as local adaptations to the environment, contributing to current populations in lower B.C. (Landis & Evans, n.d.). However, more strains may need to be tested before actually implementing the control on a large scale , as it may have an effect on native plant species (Enright & Cipollini 2007).
We found that the rosettes to flowering plants transition was the vital rate that most frequently had the highest elasticity for growing garlic mustard populations. The only variable that targeted this stage transition was increased sunlight. However, Figure 2 shows the increase in growth rate for populations simulating more sunlight was barely noticeable. This is likely because the difference in sunlight between the two regions was only 3.01%. Furthermore, garlic mustard prefers to establish in shaded regions whereas our model assumes they are in unshaded areas and receive all available light throughout the day to simplify calculations. We assumed that the transition with the highest elasticity would correspond with the variable with the largest change in growth rate, but this was incorrect because we did not account for the regional differences in the variables being that insignificant.
There were several limitations in our study due to the nature of it being a meta-analysis of existing research. Although there is a large collection of literature written about Alliaria petiolata, most focus on the effects of the plant on their surroundings rather than testing what environmental factors affect the plant’s distribution. This made it difficult for us to find suitable experimental research to base our study off of, and our selection was limited to experiments with statistically significant relationships. As a result, we only had four variables to include in our study which is not representative of the suite of the known and unknown factors that are likely affecting garlic mustard distribution across North America. Our results indicate that lower B.C. will have a growing population of garlic mustard, but it is very likely that we have missed other environmental variables that may be influential enough to make the growth rate shrink or grow even more. The limited number of articles that we could find also introduced uncertainty because our results were reliant on one study per variable.
Each variable was reliant on the relationships found from one study, meaning our meta-analysis sample size was very small. In an ideal meta-analysis, we would have several experimental results to base our simulations off of to capture the full range of possible relationships, especially for spatially dependent phenomena such as temperature and precipitation. Since these experiments were conducted in different locations (eg. the temperature experiment used seeds from Illinois, whereas the light study was conducted in Minnesota), the relationships between the variable and population response may differ when conducted at another site. This is especially important when it has been found that local adaptations may be driving responses to germination and seed bank dynamics in garlic mustard populations in North America (Blossey et al. 2017). Additionally, spatial variation within regions is another factor that we did not account for.
Our study used a “lumped” model where one value represented an entire region, even though these variables realistically vary across space. For simplicity’s sake, we designated Chicago, Illinois as our reference point for the Midwest region that we based our sunlight and precipitation calculations on. Garlic mustard populations in Chicago certainly do not experience the same precipitation that a population in St. Louis, Illinois might, but it would be computationally complicated to account for local climatic conditions for each of the 24 matrix populations we used. Chicago was chosen because it centers the overall distribution of garlic mustard sample matrices. Furthermore, the variables also depend on site locations. For example, light availability will depend on canopy cover, which varies spatially, but our model assumes each population will receive all available daylight. If we had the time and coding knowledge, a more accurate way to represent variation in the variables would be to divide the Midwestern matrices into sub-regional groups (eg. averaging similar climate station data).
Using 24 matrices based on garlic mustard populations found in the North American Midwestern region, we simulated the theoretical population growth rates in lower British Columbia based on changes in temperature, precipitation, sunlight and powdery mildew infection. Based on our results, we found that the population growth rates of garlic mustard in lower B.C. are slightly lower than those in the Midwest. We found temperature substantially limited population growth in lower B.C., which validated our first hypothesis. Meanwhile, powdery mildew, precipitation, and sunlight had positive or minimal impacts. Our second hypothesis was also accepted, as the cumulative effect of all variables decreased population growth rates in lower B.C. compared to the Midwest. There are still many unknowns regarding garlic mustard distribution, meaning we need to keep looking for more potential factors to increase our model accuracy. However, it would still be worthwhile to use this method to study historic distributions of garlic mustard, where researchers have already measured the population growth rates and environmental influences. Matrix population models are a valuable tool for predicting population responses to environmental changes and for determining which demographic stage transitions are critical to be targeted in invasion control. Furthermore, in light of anthropogenic climate change it would also be interesting to run the simulation based on predicted future environmental conditions.
References
Anderson, R. C., S. S. Dhillion, & T. M. Kelley. 1996. Aspects of the ecology of an invasive plant, garlic mustard (Alliaria petiolata), in central Illinois. Restoration Ecology 4: 181-191.
Anderson, R. C., and T. M. Kelley. 1995. Growth of Garlic Mustard (Alliaria petiolata) in Native Soils of Different Acidity. Transactions of the Illinois State Academy of Science 88:91–96.
Betz, E. C., and Z. K. Punja. 2019. Managing powdery mildew of wasabi, caused by Erysiphe cruciferarum, using biological control agents and organic fungicides. The Canadian Phytopathological Society 41:507–512.
Blossey, B., V. Nuzzo, A. Davalos. 2017. Climate and rapid local adaptation as drivers of germination and seed bank dynamics of Alliaria petiolata (garlic mustard) in North America. Journal of Ecology 105:1485–1495.
Cavers, P. B., M. I. Heagy, and R. F. Kokron. 1979. The Biology of Canadian Weeds. 35. Alliaria petiolata (M. Bieb.) Cavara and Grande. Canadian Journal of Plant Science 59:217–229.
Ciola, V., and D. Cipollini. 2011. Distribution and Host Range of a Powdery Mildew Fungus Infecting Garlic Mustard, Alliaria petiolata, in Southwestern Ohio. The American Midland Naturalist 166:40–52.
Cipollini, D., and B. Gruner. 2006. Cyanide in the Chemical Arsenal of Garlic Mustard, Alliaria petiolata. Journal of Chemical Ecology 33:85–94.
City of Burnaby. (n.d.). Invasive Species Garlic Mustard Fact Sheet. https://www.burnaby.ca/Assets/city+services/policies+projects+and+initiatives/environment/Garlic+Mustard+Fact+Sheet.pdf. Accessed March 18, 2020.
City of Victoria. 2010. Invasive Alien Alert Garlic Mustard (Alliaria petiolata). https://www.victoria.ca/assets/Departments/Parks~Rec~Culture/Parks/Documents/invasive-species-garlic-mustard.pdf. Accessed March 18, 2020.
Compadre. (n.d).. Alliaria petiolata. [Matrix] Retrieved from https://compadre-db.org/Species/46616. Accessed March 12, 2020.
Cruden, R., A. McClain, & G. Shrivastava. 1996. Pollination Biology and Breeding System of Alliaria petiolata (Brassicaceae). Bulletin of the Torrey Botanical Club 123:273-280.
Enright, S. M., & D. Cipollini. 2007. Infection by powdery mildew Erysiphe cruciferarum (Erysiphaceae) strongly affects growth and fitness of Alliaria petiolata (Brassicaceae). American Journal of Botany 94:1813–1820.
Evans, J. A., A. S. Davis, S. Raghu, A. Ragavendran, D. A. Landis, & D. W. Schemske. 2012. The importance of space, time, and stochasticity to the demography and management of Alliaria petiolata. Ecological Applications 22:1497-1511.
Footitt, S., Z. Huang, H. Ölcer-Footitt, H. Clay, and W. E. Finch-Savage. 2018. The impact of global warming on germination and seedling emergence in Alliaria petiolata, a woodland species with dormancy loss dependent on low temperature. Plant Biology 20:682–690.
Kottek, M., J. Grieser, C. Beck, B. Rudolf, and F. Rubel. 2006. World Map of the Köppen-Geiger climate classification updated. Meteorologische Zeitschrift 15:259–263.
Landis, D., and J. Evans. (n.d.). Management Options. Michigan State University. https://www.canr.msu.edu/ipm/invasive_species/garlic_mustard/management_options.
Merow, C., S. T. Bois, J. M. Allen, Y. Xie, and J. A. Silander. 2017. Climate change both facilitates and inhibits invasive plant ranges in New England. Proceedings of the National Academy of Sciences 114:3276-3284.
NOAA. (n.d.). Climate data online.[Temperature and precipitation] Retrieved from https://www.ncdc.noaa.gov/cdo-web/. Accessed March 20, 2020.
Nuzzo, V. A. 1991. Experimental Control of Garlic Mustard [Alliaria Petiolata (Bieb.) Cavara & Grande] in Northern Illinois Using Fire, Herbicide, and Cutting. Natural Areas Journal 11:158–167.
Nuzzo, V. A. 1993a. Current and historic distribution of garlic mustard (Alliaria petiolata) in Illinois. The Michigan Botanist 31:23–33.
Nuzzo, V. A. 1993b. Distribution and spread of the invasive biennial Alliaria petiolata (garlic mustard) in North America. Biological pollution: the control and impact of invasive exotic species 127-146.
Parmesan, C., and G. Yohe. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37-42.
Phillips-Mao, L., D. L. Larson, & N.R. Jordan. 2014. Effects of Native Herbs and Light on Garlic Mustard (Alliaria petiolata) Invasion. Invasive Plant Science and Management 7: 257–268.
Prati, D., and O. Bossdorf. 2004. Allelopathic inhibition of germination by Alliaria petiolata (Brassicaceae). American Journal of Botany 91:285–288.
Raghu, S., & S.L. Post. 2008. Cold Stratification Requirements for Germination ofAlliaria petiolata. Invasive Plant Science and Management 1:315–318.
Rodgers, V. L., K. A. Stinson, and A. C. Finzi. 2008. Ready or Not, Garlic Mustard Is Moving In: Alliaria petiolata as a Member of Eastern North American Forests. BioScience 58:426–436.
Stinson, K., S. Kaufman, L. Durbin, and F. Lowenstein. 2007. Impacts of Garlic Mustard Invasion on a Forest Understory Community. Northeastern Naturalist 14:73–88.
Stocker, T.F., D. Qin, G.-K. Plattner, M. Tignor, S.K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex and P.M. Midgley (eds.). 2013. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA.
TimeandDate. (n.d.). Vancouver and Chicago daytime data. Retrieved from: https://www.timeanddate.com/sun/. Accessed March 20, 2020.
Vincent, L., X. Zhang, É. Mekis, H. Wan, and E. Bush. 2018. Changes in Canada's Climate: Trends in Indices Based on Daily Temperature and Precipitation Data. Atmosphere-Ocean 56:332–349.
Welk, E., K. Schubert, and M. H. Hoffmann. 2002. Present and potential distribution of invasive garlic mustard (Alliaria petiolata) in North America. Diversity and Distributions 8:219–233.
Learning Significance